Medical Product Marking for Traceability

Medical product traceability is critical to the safety of the medical product supply chain. The unique device identifiers (UDIs) required of medical devices enable device tracking to provide for part tracking during recalls, integration with electronic health records and ensuring the security and safety of the supply chain.
We at Columbia Marking Tools are proud to be doing our part to help the country get through this difficult time. CMT remains open and operating, offering a wide range of direct part marking equipment that is critical for medical product traceability.
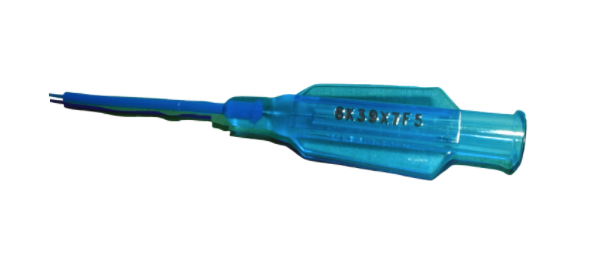
CMT marking machines used for DPM direct part marking of medical
devices includes:
- Hot stamping of product information
- Laser marking of 2D codes or machine readable information
- Impact markers for quality control and station ID
- Dies and stamps for date codes and lot numbers
Keeping manufacturing going is critical to winning our fight against this virus. At CMT, we are happy to be able to do our part.
For more information or quotes please email quotes@columbiamt.com


